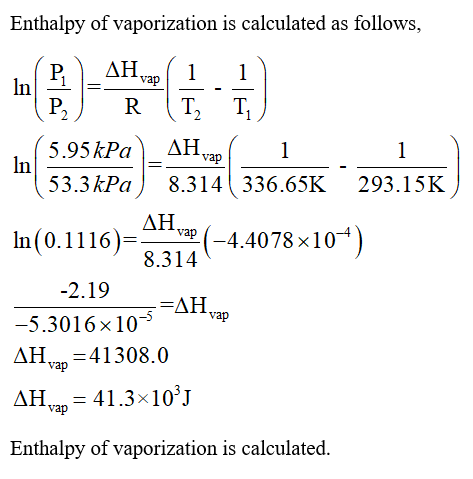
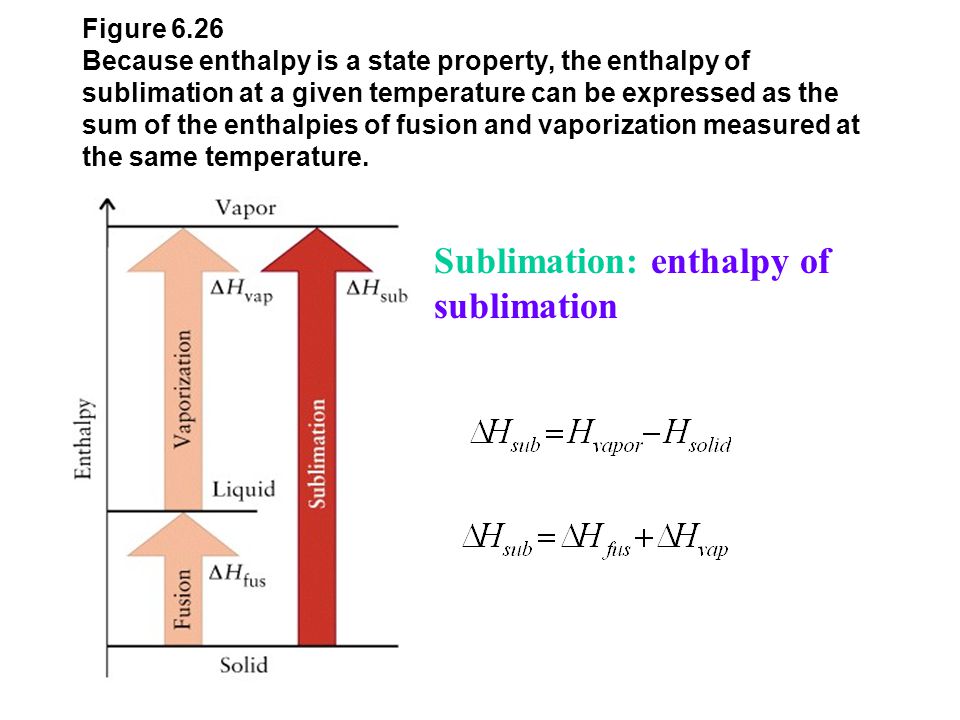
SciELO - Brasil - Enthalpy of mixing and heat of vaporization of ethyl acetate with benzene and toluene at 298.15 k and 308.15 k Enthalpy of mixing and heat of vaporization of

Using Heat of Fusion or Vaporization to Find the Heat Needed to Melt or Boil a Substance | Chemistry | Study.com
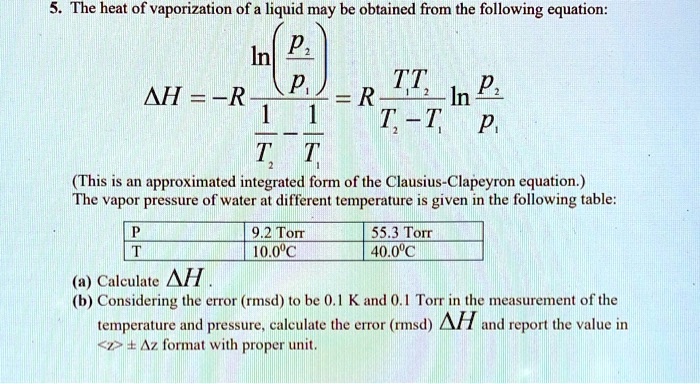
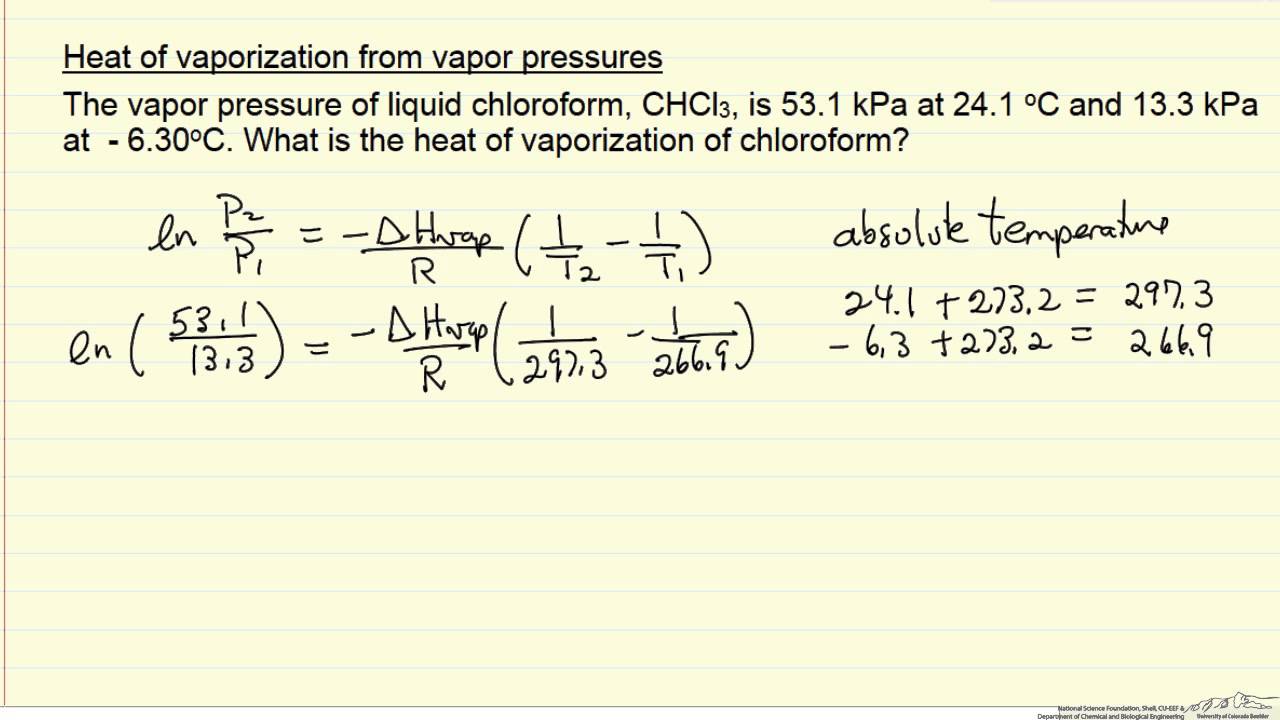
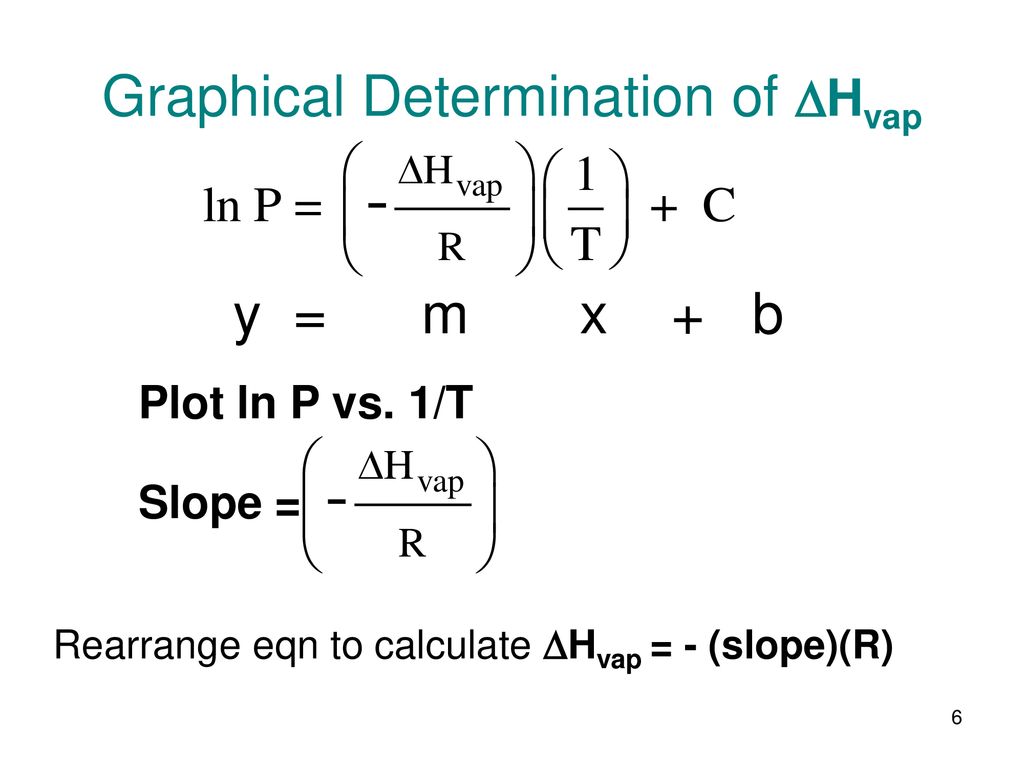
SOLVED: The heat of vaporization of a liquid may be obtained from the following equation: ΔH = -R * ln(P2/P1) * T1 / (T2 - T1) (This is an approximated integrated form
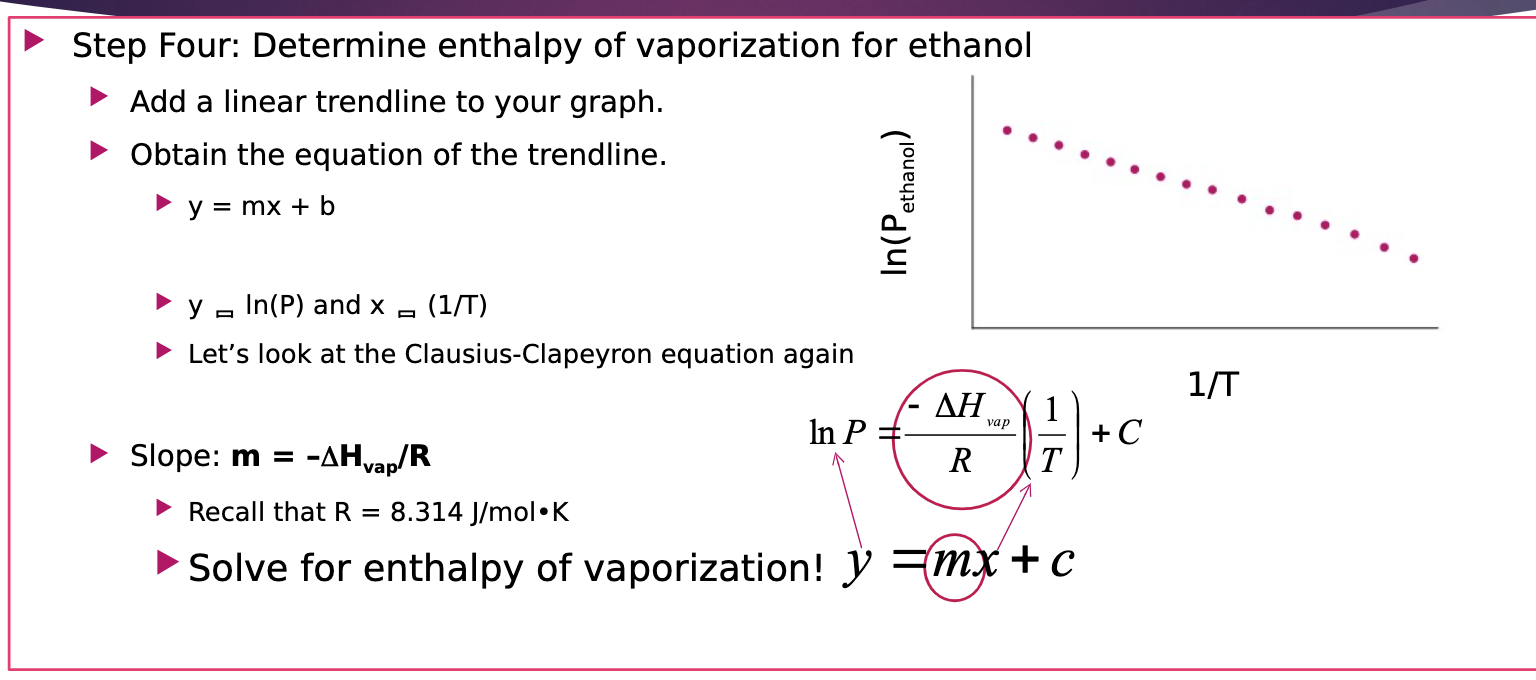
88. The values for delta H vap.and delta S vap. for ethanol are respectively 38.594 kJ/mol and 109.8 J/K. What will be the boiling point of ethanol ?
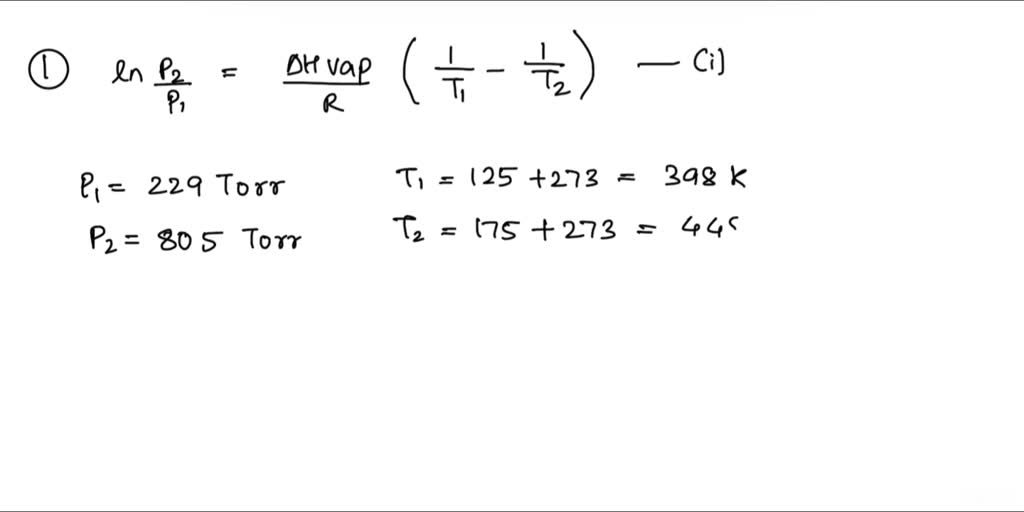

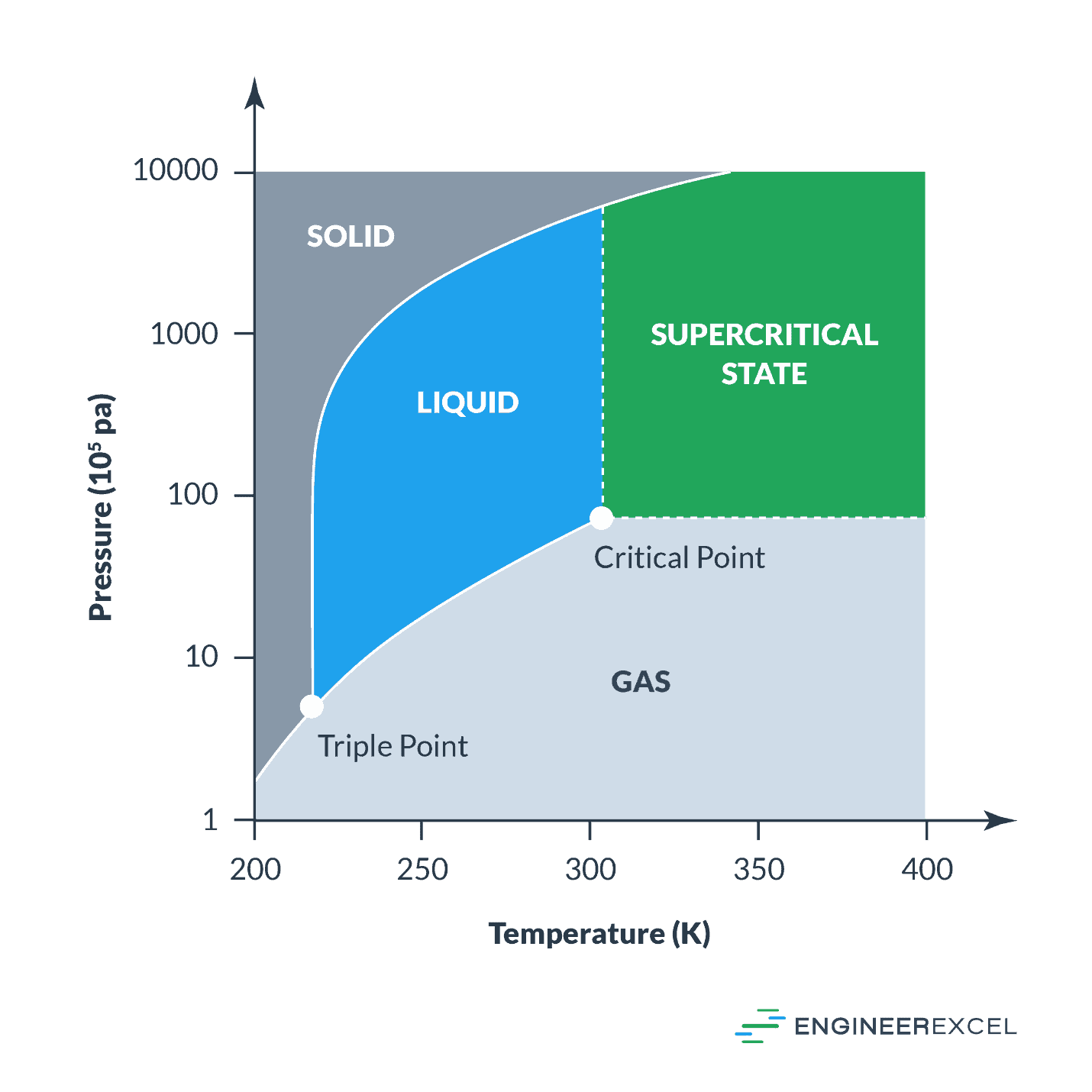

-438.png)